Fast block to polyspermy
Explainer for our 2018 manuscripts published in the Journal of General Physiology
Fertilization of an egg by more than one sperm presents one of the earliest barriers to successful reproduction. Fertilization by multiple sperm, a condition known as polyspermy, leads to gross chromosomal abnormalities and is lethal to the developing embryos. Due to these catastrophic consequences, eggs employ multiple processes that protect the protect the nascent embryo. The two most widely studied are called the fast and slow blocks to polyspermy. As their names imply, these blocks occur at different times to fertilization. In the slow block to polyspermy, eggs release material into the extracellular space, and within minutes of its release, this material transforms the egg from a gamete ready for sperm entry, to a structure unable to permit fertilization. The slow block is used by eggs from nearly all sexually reproducing animals. By contrast, the fast block to polyspermy is mainly used by eggs from external fertilizers.
In the fast block, fertilization activates a depolarization of the egg. By currently unknown mechanisms, sperm can bind to, but will not enter, a depolarized egg. Although fertilization-signaled depolarizations have been recorded for many years, the signaling pathways that activate the fast block, including the molecular identity of the channel that conducts the depolarizing currents, was not yet known for any species. We sought to uncover the identity of the channel opened at fertilization of eggs from the African clawed frog, Xenopus laevis, and the pathway by which the channel is activated.
Previous experiments established that a Cl- current depolarizes frog eggs at fertilization. Frogs fertilize in pond water, which is more dilute than the intracellular milieu; when a Cl- channel opens, the anion leaves the cell to make the membrane more positive. Separate experiments revealed that an increase in intracellular Ca2+ is required for X. laevis eggs to depolarize at fertilization. Based on these two pieces of data, we sought to identify the Ca2+-activated Cl- channels present in X. laevis eggs.
We turned to bioinformatics to uncover which ion channels are present in the fertilization-competent eggs and identified two Ca2+-activated Cl- channels in the X. laevis egg: xTMEM16A and xBest2A. With two candidate channels in hand, we next sought to uncover pharmacologic tools that would enable us to discriminate between currents carried by each. We identified two compounds that effectively inhibited xTMEM16A while having only marginal effects on xBest2A: Ani9 and MONNA. Unfortunately, we were unable to identify compounds that targeted xBest2A without also blocking xTMEM16A.
Typical depolarization recorded from an X. laevis egg during fertilization. The dashed line denotes 0 mV, and the scale bar reflects 10 s on the x-axis, and 5 mV on the Y-axis.
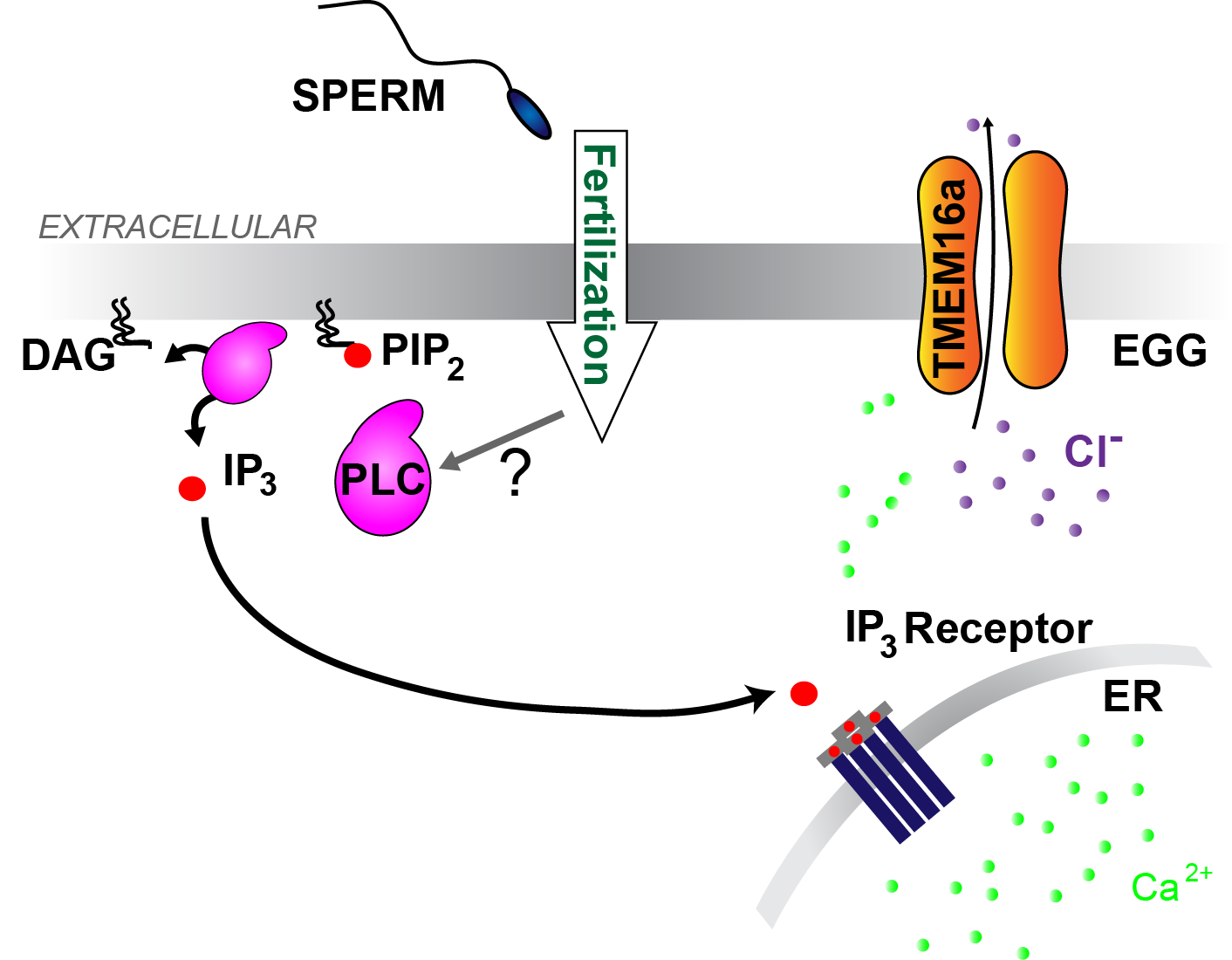
The fast block to polyspermy in X. laevis eggs is activated by PLC induced production of IP3, Ca2+ release from the ER, and opening of TMEM16A channels.
By comparing whole cell recordings from X. laevis eggs during fertilization under control conditions or in the presence of Ani9 or MONNA, we found that block of xTMEM16A either significantly slowed, or completely inhibited, the fertilization-evoked depolarizations. These data indicate that fertilization of X. laevis eggs activated xTMEM16A channels to depolarize the egg during the fast block to polyspermy (Wozniak et al. 2018a).
After establishing that opening of the the Ca2+-activated Cl- channel signals the fast block in X. laevis, we sought to uncover the source of Ca2+ that activates these channels. Although X. laevis eggs express the Ca2+-permeant TrpV4 channel, we found that Ca2+ entry is not required for the fast block.
We next turned to our alternate hypothesis, that Ca2+ release from an intracellular store initiates signals opening of TMEM16A channels. Indeed, block of the IP3 receptor or phospholipase C (PLC) prevented any fertilization-evoked depolarization (Wozniak et al. 2018b).
Together our data reveal that fertilization of X. laevis eggs activates a PLC to increase IP3, evoke a Ca2+ release from the ER, to then activate xTMEM16A channels and depolarize the egg.